Basic Info.
Model NO.
Saliva Collection Device
Group
Human
Features
Home Saliva Testing Kit, Plastic Tube Receptacle
MOQ
10000
Product Name
Saliva Collection Funnel in Medical or Lab
Saliva Sample Kit
Saliva
Other Name
Saliva Funnel, Saliva Device
Transport Package
Cartons
Specification
50pcs/box
Trademark
Runmei
Origin
China
HS Code
1108966068
Production Capacity
100, 000 Boxes / Day
Product Description
FDA CE ISO 13485 Approved quick and easy Split Sample Saliva Collection Device


Product Description
The Single-use Samplers of Hunan Runmei Gene Technology Co., Ltd. is improved from the Hanks virus preservation solution, which have different versions of formulas for different markets and customized requests. Compared with the traditional Hanks virus preservation solution, it has a better ability to maintain the integrity of the virus. At the same time, the positive rate of PCR detection and the positive rate of virus isolation are higher than the traditional Hanks virus preservation solution. It can be widely used in the collection and transportation of clinical influenza,avian influenza, hand, foot and mouth disease, measles and other virus specimens, as well as chlamydia, mycoplasma, urea-plasma specimens.
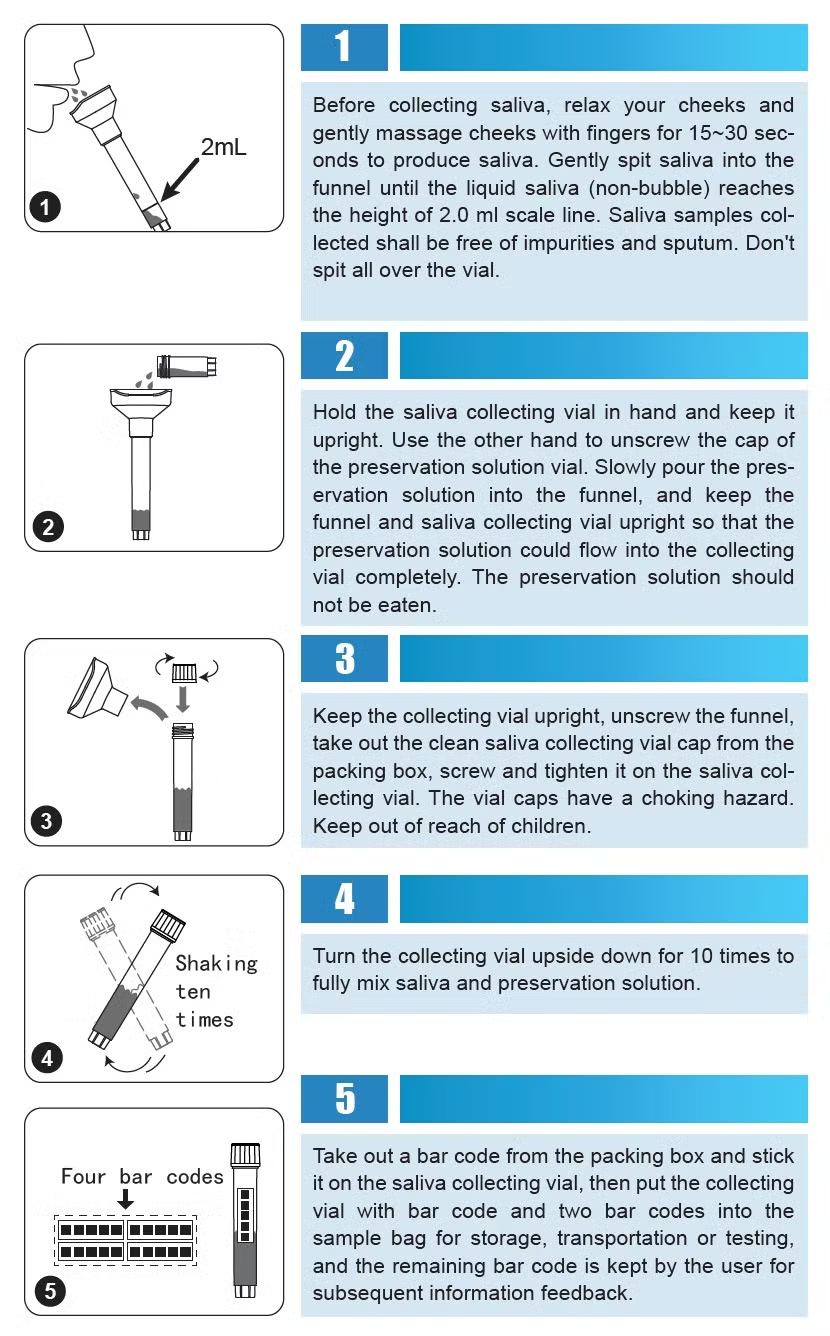
Intended Use of Disposable Sampler
It is used for the collection and transportation of clinical influenza, avian influenza, hand, foot and mouth disease, measles and other virus specimens, as well as chlamydia, mycoplasma and urea-plasma specimens.
Main Components
Storage Conditions
Store at normal temperature 5 -25°C.
Applicable sample types: Saliva.
Storage and transportation of samples: After the sample is collected, it should be transported to the corresponding laboratory for testing within 96 hours at regular storage temperature. It can preserve DNA, RNA and antigens of bacteria, viruses, and Chlamydia for five days when stored at regular storage temperature (20 - 37°C); 7 days if stored at 4°C and up to 6 months when stored at -20°C or -70°C. The sample should avoid repeated freezing and thawing.
Precautions
1. Single-use samplers should be sent for inspection as soon as possible after sampling, and immediately be transported at a low temperature of 2-8°C. The collected samples can be stored at 2-8 °C for a short period of 48 hours, long-term storage should be placed in low temperature conditions below -20°C, -70°C or store at -196°C
2. This single use sampler is strictly prohibited for the sampling of bacterial samples. The antibiotics contained in the preservation solution itself have an inhibitory effect on bacteria.
3. It is forbidden to directly touch the collected patient, or to sample the patient after wetting the swab.
4. Sampling should be carried out in strict accordance with the sampling procedure, so that the sampling location is accurate and the sampling strength is uniform and suitable, otherwise it will affect the quality of sample collection.
5. Don't use the disposable sampler after the expiration date or the product packaging is damaged.
Expiration Data
12 months
Company Profile:
Hunan Runmei Gene Technology Co., Ltd is a high-tech enterprise dedicated to the
development of the gene detection products and the construction of big data service
platforms led by a team of doctors at home and abroad. Our company's strategic goal is
to base on China and radiate the world, and to solve the pain points and difficulties of the
industry and create value for human beings as our corporate purpose. At present, our
company has completed the construction of product systems for pathogen biology fluoresence
quantitative PCR detection kits, pathogen biology ELISA detection kits and pathogen biology
immune colloidal gold detection kits.



Certification:




Thank you for our Canadian customer's 5 star Review:




Product Description
The Single-use Samplers of Hunan Runmei Gene Technology Co., Ltd. is improved from the Hanks virus preservation solution, which have different versions of formulas for different markets and customized requests. Compared with the traditional Hanks virus preservation solution, it has a better ability to maintain the integrity of the virus. At the same time, the positive rate of PCR detection and the positive rate of virus isolation are higher than the traditional Hanks virus preservation solution. It can be widely used in the collection and transportation of clinical influenza,avian influenza, hand, foot and mouth disease, measles and other virus specimens, as well as chlamydia, mycoplasma, urea-plasma specimens.

Saliva collection funnel's Benifits
- Improve donor care and compliance with painless, non-invasive sample collection
- Eliminate phlebotomy costs
- Ideal for use with children or patients that will not comply with blood collections
- Increase efficiency, minimize sample handling and reduce handling errors with a compatible format for high-throughput processing
- Sample remains stable for years at room temperature, reducing transportation and storage costs
- Sample can be mailed using the standard postal system
- DNA from saliva is equivalent to DNA from blood for downstream applications
Intended Use of Disposable Sampler
It is used for the collection and transportation of clinical influenza, avian influenza, hand, foot and mouth disease, measles and other virus specimens, as well as chlamydia, mycoplasma and urea-plasma specimens.
Main Components
| Number | Composition | 20 Testing Kits/box |
| 1 | Collection Funnel(with a collection tube) | 20 pcs/box |
| 2 | Saliva Preservation Tube(with 3ml saliva preservation solution ) | 20 pcs/box |
| 3 | Biohazard Bag | 20 pcs/box |
| 4 | Manual | 1 serving/box |
Storage Conditions
Store at normal temperature 5 -25°C.
Applicable sample types: Saliva.
Storage and transportation of samples: After the sample is collected, it should be transported to the corresponding laboratory for testing within 96 hours at regular storage temperature. It can preserve DNA, RNA and antigens of bacteria, viruses, and Chlamydia for five days when stored at regular storage temperature (20 - 37°C); 7 days if stored at 4°C and up to 6 months when stored at -20°C or -70°C. The sample should avoid repeated freezing and thawing.
Precautions
1. Single-use samplers should be sent for inspection as soon as possible after sampling, and immediately be transported at a low temperature of 2-8°C. The collected samples can be stored at 2-8 °C for a short period of 48 hours, long-term storage should be placed in low temperature conditions below -20°C, -70°C or store at -196°C
2. This single use sampler is strictly prohibited for the sampling of bacterial samples. The antibiotics contained in the preservation solution itself have an inhibitory effect on bacteria.
3. It is forbidden to directly touch the collected patient, or to sample the patient after wetting the swab.
4. Sampling should be carried out in strict accordance with the sampling procedure, so that the sampling location is accurate and the sampling strength is uniform and suitable, otherwise it will affect the quality of sample collection.
5. Don't use the disposable sampler after the expiration date or the product packaging is damaged.
Expiration Data
12 months
Company Profile:
Hunan Runmei Gene Technology Co., Ltd is a high-tech enterprise dedicated to the
development of the gene detection products and the construction of big data service
platforms led by a team of doctors at home and abroad. Our company's strategic goal is
to base on China and radiate the world, and to solve the pain points and difficulties of the
industry and create value for human beings as our corporate purpose. At present, our
company has completed the construction of product systems for pathogen biology fluoresence
quantitative PCR detection kits, pathogen biology ELISA detection kits and pathogen biology
immune colloidal gold detection kits.



Certification:




Thank you for our Canadian customer's 5 star Review:


